![]()
Glaxo-Wellcome have just released details of their phase II studies into "Dutasteride (GI198745)" a dual 5-alpha-reductase inhibitor at the American Academy of Dermatology (AAD) meeting in San Francisco.
Propecia (Finasteride), the latest approved medication for hair loss, inhibits one of the two types of this enzyme which turns Testosterone into DHT. Dutasteride, the medication discussed in this article, blocks both types of the enzyme and so it is believed it will be more effective at treating hair loss.
The Phase II studies involved 416 men aged 21-45 with Norwood patterns IIIv, IV, and V. The study lasted for 6 months and was randomized and double-blind (meaning the investigators did not know who was getting which medication). Placebo and Proscar were compared to 0.05, 0.1, 0.5, and 2.5mg of Dutasteride. Proscar (the 5mg form of Finasteride - Propecia is the 1mg form) was used because Propecia was not available at the time the studies started.
Very little Dutasteride is required to inhibit type 1 5-alpha-reductase but very large quantities of Propecia are required to do so. For type 2 5-alpha reductase (the one Propecia blocks), even less Dutasteride is required to block it than Propecia. So small doses of Dutasteride provide very good inhibition of both types of the 5-alpha reductase enzyme.
There were no results presented on efficacy but sexual side effects have occurred in the studies.
Dutasteride at this point remains on hold for Phase III studies. It is unknown why the company has not decided yet whether to proceed with Phase III trials. The two most likely reasons may be that either the company believes the drug will not make enough money if released (Propecia sales have been described as disappointing in several press articles evaluating sales) or that the company does not believe the treatment will be approved for the cosmetic purpose of hair loss due to side effects. Glaxo-Wellcome made a statement that a decision would be made in February regarding the trials, however to date no decision has been publicly announced.
The good news is that Phase III trials have already been completed for Benign Prostatic Hyperplasia (BPH, also known as enlarged prostates) and the drug is scheduled to be submitted to the FDA for approval sometime this year and approved and marketed sometime next year. Finasteride followed a similar course and was first approved for BPH and then later for hair loss.
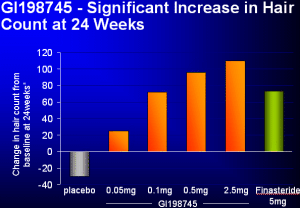 Well folks, your patience is starting to
pay off. A new treatment which has been in the sights of many hair loss
sufferers for many months has officially proven itself more effective than
Propecia at maintaining and regrowing hair. The Chart to the left compares
Dutasteride's hair count results (Orange Bars) to 5 times the normal Propecia
dosage, and the hair counts it returns (Green Bar). As you can see, though the
difference may seem only marginal, Dutasteride has proven itself superior, as a
0.1mg dosage can equal 5 times the Propecia dosage in effect on hair count.
Well folks, your patience is starting to
pay off. A new treatment which has been in the sights of many hair loss
sufferers for many months has officially proven itself more effective than
Propecia at maintaining and regrowing hair. The Chart to the left compares
Dutasteride's hair count results (Orange Bars) to 5 times the normal Propecia
dosage, and the hair counts it returns (Green Bar). As you can see, though the
difference may seem only marginal, Dutasteride has proven itself superior, as a
0.1mg dosage can equal 5 times the Propecia dosage in effect on hair count.